Posted: April 12, 2019 | Author: 3Rs in Ecology | Filed under: 2019, ERES525, Module Critique Assignment | Tags: apexpredator, conservation, Ecological theories, habitat, habitatfragmentation, mesopredator, mesopredatorreleasetheory, restoration ecology, Trophic Cascade |
By Alice Hales
Abstract
Mesopredator release theory involves the decline or absence of an apex predator, causing a drastic increase in mesopredator populations after the release from top-down control (11). This increased predation and competition then results in the decline or extinction of a wide range of prey species (8). This theory is applicable worldwide and has many implications in the conservation or restoration of ecosystems (4). It is becoming more apparent that apex predators are a key component of sustaining biodiversity and functional systems (6). Conservation strategies are being informed by the theory to mitigate negative effects. These can include reintroduction of an apex predator, control of mesopredators, expanding prey habitats to increase abundance and restricting factors that could facilitate growing mesopredator numbers (6,8). As an alternative outcome to mesopredator release, occasionally the loss of a top predator causes an increased in shared prey abundance (2).
Introduction
Mesopredators are an essential element of many ecosystems by regulating prey species too small for larger predators (11). They occupy the middle trophic levels, being smaller in size relative to apex predators (11). The food web of a system determines their role and density; often they are controlled by apex predators with top-down control, or by food availability from the bottom up (3). However, some situations can lead to population explosions of mesopredators (8). The mesopredator release theory describes the trophic cascade within an ecosystem following the removal or absence of a higher predator (8). A trophic cascade is the suppression of a trophic level resulting in the ecological release of another (8). Mesopredators become increasingly abundant in the lack of top-down control; often resulting in dramatic decreases, or extinction, of prey populations (4). This can have multiple follow on effects, changing prey abundance and functionality of the ecosystem. This theory influences conservation decisions regarding community recovery and the methods carried out. In addition, occasionally the loss of an apex predator results in increased shared prey abundance, rather than mesopredator release (2).
Mesopredator Release: Cause, Effect & Action
Examples of mesopredator release expand throughout the globe. In America, the loss of wolves (Canis lupus) could cause a rapid increase in the populations of coyotes (Canis latrans); endangering a wide array of smaller vertebrates (5). Without wolves controlling coyote numbers, signs of mesopredator release are evident. No longer under the restrictions of territory or food availability, the coyote population soon grew to high numbers. Dramatic increase of predation on coyote prey puts strain on species of rodents, ungulates and birds (5). This includes some taxa notably threatened under the U.S. Fish and Wildlife Service (USFW) list (5).
Conservation efforts to facilitate community recovery began by poison baiting to cull coyote populations (5). This is a conflicting strategy for many in terms of animal welfare, and has the potential to cause conflict among communities (5). In the absence of wolves, higher large ungulate populations are expected. This can increase food opportunities for coyote via carrion. To mitigate negative impacts of mesopredator release in this case, wolf population restoration, restricting external food sources for coyotes and focusing on declining small prey populations are the best solutions (5). In Yellowstone, there was a 39% decline in coyote numbers post wolf restoration; a preferred alternative to using poison for mesopredator control (5).
Tigers (Panthera tigris) in the forests of Asia are apex predators, with leopards (Panthera pardus) and dholes (Cuon alpinus) as the mesopredator species (10). A study showed that the intermediate species distribution is governed by the territory of the apex predator, not where the most prey is (10). This evidence for potential mesopredator release highlights the importance of maintaining top predator populations to sustain biodiversity (10). In the absence or severe decline of tigers, dholes and leopards will grow to outnumber them (10). This sudden population surge will cause the decline of ungulate and primate prey species; threatening prey existence as well as the remaining tigers (10).
The threat to tigers and prey populations has triggered restoration attempts for the system. Open grasslands and new feeding habitats created for ungulate prey increases their abundance (10). An increase in large prey and ideal open hunting habitat for tigers will benefit their populations despite the potential of being outnumbered by mesopredators (10). Maintaining a sustainable level of prey species, the functionality of the system will be conserved (10). This is positive strategy for conservation and less invasive than having to manually control mesopredator numbers. Though the limitations of expanding or altering habitat is encroaching human civilisation, disturbance of other species and risk of the solution being temporary.
Declining Apex Predators
As habitat loss and fragmentation becomes more apparent, all large predator ranges are contracting, while mesopredator ranges are rapidly expanding (3). These fragmented landscapes often border human communities; putting large predators at risk, increasing edge effects and disrupting biological function (1). Habitat loss effects whole ecosystems, less large prey and territory for bigger predators can cause mesopredator release (7).
Conservation and Alternative Outcomes
Crooks & Soule state that many conservation efforts have been put in place to protect larger carnivores due to this theory (9). Originally persecuted for endangering humans, people are now fighting for the protection of apex predators to avoid significant ecosystem shifts (3). Community recovery and maintaining trophic levels in ocean and terrestrial systems has become imperative (9).
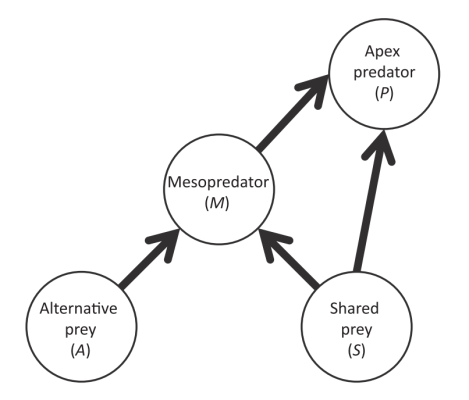
Figure 1. Model of interactions between apex predator, mesopredator, shared prey and alternative prey. Loss of apex predator can result in increased shared prey.
As an alternative to mesopredator release, occasionally reduction or loss of an apex predator can be beneficial for prey animals (2). In systems where mesopredators have both shared prey (with apex predators) and alternative prey; shared prey populations increase, even with high mesopredator abundance (2). Figure 1 illustrates the model of interactions between predator and prey levels. For instance, on an island system, removal of cats (top predator) resulted in an increase in seabirds; this is despite the high rat (mesopredator) abundance (2). To ensure mesopredator release does not have a negative impact on prey species, two management options are suggested (2). Selective control of the apex predator and mesopredator, or controlling the apex predator and alternative prey, will result in a rise of shared prey density (2). Apex predators control mesopredators even in low densities, reducing pressure on shared prey (2).
Conclusion
Mesopredator release is the trigger for countless ecosystem changes (6, 11). Increasing habitat loss and fragmentation is the leading cause of the decline, movement or extinction of apex predator species (1). With the loss of the top-down, mesopredators are no long restricted by food availability or territory and undergo population explosions (8). The effects of the mesopredator release theory reach all corners of conservation, especially regarding loss of prey biodiversity and abundance in these systems (2).
Conservation has strong focus on the protection of these large predators and reserving their habitat to reduce mesopredator release (6). Strategies are appearing to mitigate negative effects; reintroducing apex predators to damaged systems, expanding prey habitats to increase populations, controlling mesopredators and preserving natural control (6). Occasionally systems respond to losing a top predator by increasing shared prey populations, even if mesopredators are present (2). Mesopredator release theory informs conservation strategies, offering insight into the relationship between trophic levels and how negative effects can be combatted.
References
- Newsome, T. M., Greenville, A. C., Ćirović, D., Dickman, C. R., Johnson, C. N., Krofel, M., … Wirsing, A. J. (2017). Top predators constrain mesopredator distributions.Nature communications, 8.
- Nishijima, S., Takimoto, G. & Miyashita, T. (2014). Roles of Alternative Prey for Mesopredators on Trophic Cascades in Intraguild Predation Systems: A Theoretical Perspective. The American Naturalist, 183(5), 625-637.
- Pruh, L. R. et al. (2009). The Rise of the Mesopredator. Bioscience, 59(9).
- Rayner, M. J., Hauber, M. E., Imber, M. J., Stamp, R. K. & Clout, M. N. (2007). Spatial heterogeneity of mesopredator release within an oceanic island system. Proceedings of the National Academy of Sciences, 104(52), 20862-20865.
- Ripple, W. J., Wirsing, A. J., Wilmers, C. C. & Letnic, M. (2013). Widespread mesopredator effects after wolf extirpation. Biological conservation, 160, 70-79.
- Ritchie, E. G., Elmhagen, B., Glen, A. S., Letnic, M., Ludwig, G. & McDonald, R. A. (2012). Ecosystem restoration with teeth: what role for predators? Trends in Ecology and Evolution, 27(5).
- Ritchie, E. G. & Johnson, C. N. (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecology letters, 12, 982–998.
- Soule, M., Bolger, D., Alberts, A. C., Wrights, J., Sorice, M. & Hill, S. (1988). Reconstructed Dynamics of Rapid Extinctions of Chaparral-Requiring Birds in Urban Habitat Islands. Conservation Biology, 2(1).
- Soule, M. & Crooks, K. R. (1999). Mesopredator release and avifaunal extinctions in a fragmented system. Nature, 400.
- Steinmetz, R., Seuaturien, N. & Chutipong, W. (2013). Tigers, leopards, and dholes in a half-empty forest: Assessing species interactions in a guild of threatened carnivores. Biological Conservation, 163, 68-78.
- Tambling, C., Avenant N. L., Drouilly, M. & Melville, H. I. A. S. (2018). The role of mesopredators in ecosystems: Potential effects of managing their populations on ecosystem processes and biodiversity. Centre for African Conservation Ecology: South Africa.
Posted: May 4, 2014 | Author: 3Rs in Ecology | Filed under: 2014, BIOL420, Research Essay | Tags: apexpredator, Australia, barrierfence, dingo, dingoes, mesopredator |

Written by Alice Derwentsmith
The use of dingoes (Canis lupus dingo) as apex predators and biodiversity regulators in the Australian landscape has recently generated a significant amount of discussion[1]. This concept has ben extensively researched however there has yet to be any movement from the Australian Commonwealth Government on reduced culling of dingoes or a response to the practice of selectively introducing dingoes into the productive southern eastern grazing regions of Australia. This issue is multifaceted, with implementation and outcomes largely influenced by political agendas. Financial considerations and pressure from lobby groups that coerce policy makers into non-scientifically supported proposals, produce variable and poor environmental outcomes. Public perceptions that the dingo is a menace, killer, and an environmental pest are often misguided, irrational or unjustified[1], and have consequently obstructed the government from making sound environmental decisions.
Currently, Australia’s native mammal and marsupial populations are on a downwards spiral[2]. This is mainly due to introduced predators such as the cat (Felis catus) and European red fox (Vulpes vulpes)[2]. It is well known that dingoes, as apex predators, act as trophic and biodiversity regulators and have the potential to subdue feral predators such as those described above[3]. Dingoes demonstrate the ‘landscape of fear’ phenomenon towards feral cats and foxes, meaning that they avoid areas in which dingoes frequent due to fear[4]. This in turn allows smaller native mammals and marsupials to benefit from less predation effort.
The exclusion of dingoes from the southern half of the continent due to the dingo barrier fence, has led to great changes in ecosystem and biodiversity functions[3]. Research indicates that the re-introduction of dingoes into the southern half of Australia will limit the presence of feral cats, foxes and rabbits and in turn have a positive trophic cascade effect on the ecosystem[4][5].
Dingoes are currently listed as vulnerable on the IUCN Redlist list. The IUCN attempts to bring their concerns of threatened biodiversity to the attention of policy makers[7].

Figure 1. Map of Australia’s dingo barrier fence. Built in the late 1890’s early 1900’s, the fence spans 5531km across the landscape[6].
This advice and high level of concern has somewhat been ignored by the Australian Government as there has been no move to protect the species and culling still exists in many states.
With so much public interest and research being conducted, why has the Australian Government yet to do anything? This is due to a combination of factors such as money, public opinions and governmental power.
Governments are often slow to act or react, and are prone to responding to, or being influenced by, pressure or lobby groups. This resonates for the Australian Government in regards to managing many environmental issues. For instance the reintroduction of cattle into the Victorian High Country has no scientific merit, but is a reaction to an idealistic notion that can be linked to the famous Man From Snowy River poem, film, and previous heritage. Research suggests that grazing has a detrimental impact on the landscape and has no impact on fuel reduction to decrease fire risk[8][9]. The lobby group, Mountain Cattlemen, are powerful and are very much invested in this issue and therefore pressure the government. In the case of the dingo, these powerful lobby groups are farmers.
Agriculture is a large source of income for the government and local communities and takes many forms. It is a well known fact that dingoes attack livestock, with sheep being their easiest targets[10]. They can at times attack many sheep in a single visit but generally only eat one[10], leaving farmers in the position to euthanise the remaining injured sheep. Financial and emotional turmoil for the farmer is consequently ensued, which is costlyand therefore requires careful consideration when proposing to introduce an animal such as a dingo. Australia produces and exports the largest amount of wool globally[11], which provides the government with a large source of income. Protecting this income and that of the farmer weighs heavily on decision makers.
“The deliberate introduction of dingoes to control exotic predators is unlikely because they affect agricultural interests and other human activities”
– Departments of Environment, Water, Heritage and the Arts in 2008 (Feral Cat Threat Abatement Plan)[12]
Along with wool exports, Australia also participates in the live animal export trade, which earned $996.5 million dollars in 2009[13]. The notion of introducing dingoes into areas where agriculture intensity is high is not favoured by the government as it could cause have a detrimental impact on profit and the loss of farmers’ income and livelihoods.
Implementing dingoes as a pest control measure presents a major hurdle for the government in the form of legislation. Currently, there is no nationwide system that classifies dingoes into a native or non-native species, triggering each state to develop its own system, classification and subsequent management. The level of protection for dingoes varies across states. In all states and territories, with the exception of dingo free Tasmania, dingoes can legally be culled outside of national parks by one of two means: legislative sanctions or by private permit.
There is also difficulty with fact that some states or territories have legislation that contradicts or can be seen to contradict another piece of legalisation. For example, in New South Wales, dingoes are a protected species when in national parks and nature reserves under the National Parks and Wildlife Act 1974 and the Threatened Species Conservation Act 1995[14]. Once dingoes exit the national park, they become unprotected and are therefore a pest species under the Rural Lands Protection Act 1998 (Table 1)[14]. If the protection of dingoes is sought in the future, overarching federal legislation such as the Environmental Protection and Biodiversity Conservation Act 1999 along with a Threat Abatement Plan should be introduced.
Table 1. Current legal status of dingoes in Australia[14]
| State/Territory |
Classification of dingoes |
Relevant legislation |
Level of protection |
| Australian Capital Territory |
Protected species |
Nature Conservation Act 1980 |
Permit needed for culling |
| New South Wales |
Threatened species |
National Parks and Wildlife Act 1974Threatened Species Conservation Act 1995 |
Protected in National Parks |
| Pest species |
Rural Lands Protection Act 1998 |
Government responsible for management on public lands |
| Northern Territory |
Undeclared |
Parks and Wildlife Conservation Act 1993 |
Unprotected. Landholders not obligated to cull |
| Queensland |
Pest species |
Rural Lands Protection Act 1985 |
Landholders obligated to control on their land |
| South Australia |
Pest species (South of the barrier fence) |
Animal and Plant Control Board Act 1986 |
Landholders obligated to control on their land |
| Wildlife species (North of the barrier fence) |
Animal and Plant Control Commission 1993 |
Protected – level unknown |
| Tasmania |
Dingo free- No classification |
N/A |
N/A |
| Victoria |
Pest species |
Catchment and Land Protection Act 1994 |
Landholders obligated to control on their land |
| Western Australia |
Declared animals |
Agriculture and Related Resources Protection Act 1976 |
Landholders obligated to control on their land |
| Unprotected fauna |
Western Australian Wildlife Conservation Act 1950 |
Control confined to livestock areas |
There have been two recorded fatalities from dingoes in history; Azaria Chamberlain and the death of a child at Fraser Island[15]. In response to the Fraser Island attack, the Queensland Government immediately introduced a dingo culling program at Fraser Island, even though there hadn’t been a fatal attack for more than 20 years[15]. This reflects the opinion of many that dingo culling is an emotional response with little if any scientific backing.Whilst an entire legislative change would be ideal for managing the culling of dingoes and their subsequent protection, the likelihood of it happening in the near future is low. The perception of the public and farmers alike is not geared towards the reintroduction of dingoes, and implementing such proposals may alienate some voters and or polarise a portion of the electorate.
A recent example of an emotional response to an issue with the interaction between humans and their environment is the culling of sharks off the Western Australia coast. This reaction was in response to the deaths of seven beachgoers from 2010 to 2013[16]. Baited drum lines were used to hook and kill large sharks that were in close proximity to popular beaches[16]. There is little or no scientific evidence to support the view that the drum lines will deter sharks from public beaches or that there is no confounding environmental impacts, yet the government implemented the proposal.
The attachment of emotion to environmental decision makers is of great concern for many Australians. Ideally, decisions should be based on merit, with all issues or concerns given consideration and a balanced decision formulated that applies less weight to emotion and more to scientific reasoning. In the case of the dingo, their environmental benefits should be acknowledged, explored and implemented if necessary. In addition to this, attempts to save or protect native species should always be placed at a higher priority than agricultural and economic interests. In order for Australian environments and ecosystems to flourish in the future, legislation must be brought under one federal system and reflect environmental needs without the input of emotions, public perceptions, finances and governmental popularity.
References:
- Bradshaw, C. J. A., & Ritchie, E. (2012). Can Australia afford the dingo fence? The Conversation, online blog. Retrieved from http://theconversation.com/can-australia-afford-the-dingo-fence-7101
- Johnson, C. N., Isaac, J. L., & Fisher, D. O. (2007). Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proceedings of the Royal Society B: Biological Sciences, 274(1608), 341-346.
- Letnic, M., Ritchie, E. G., & Dickman, C. R. (2012). Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biological Reviews, 87(2), 390-413.
- Brook, L. A., Johnson, C. N., & Ritchie, E. G. (2012). Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. Journal of applied ecology, 49(6), 1278-1286.
- Glen, A. S., Dickman, C. R., Soulé, M. E., & Mackey, B. G. (2007). Evaluating the role of the dingo as a trophic regulator in Australian ecosystems. Austral Ecology, 32(5), 492-501.
- Newsome, A. E., Catling, P. C., Cooke, B. D., Smyth R. (2001) Two ecological universes separated by the Dingo Barrier Fence in semi-arid Australia: interactions between landscapes, herbivory and carnivory, with and without dingoes. The Rangeland Journal 23 , 71–98.
- IUCN 2013. The IUCN Red List of Threatened Species. Version 2013.2. <http://www.iucnredlist.org>.
- Williams, R. J., & Ashton, D. H. (1987). Effects of disturbance and grazing by cattle on the dynamics of heathland and grassland communities on the Bogong High Plains, Victoria. Australian Journal of Botany, 35(4), 413-431.
- Wahren, C. H. A., Papst, W. A., & Williams, R. J. (1994). Long-term vegetation change in relation to cattle grazing in sub-alpine grassland and heathland on the Bogong High-Plains: an analysis of vegetation records from 1945 to 1994. Australian Journal of Botany, 42(6), 607-639.
- Thomson, P. C. (1992). The behavioural ecology of dingoes in north-western Australia. III. Hunting and Feeding behaviour, and diet. Wildlife Research, 19(5), 531-541.
- Meale, S. J., Chaves, A. V., Ding, S., Bush, R. D., & McAllister, T. A. (2013). Effects of crude glycerin supplementation on wool production, feeding behavior, and body condition of Merino ewes. Journal of animal science, 91(2), 878-885.
- Department of the Environment, Water, Heritage and the Arts (DEWHA) (2008). Threat abatement plan for predation by feral cats, DEWHA, Canberra.
- Department of Agriculture (2014). Live animal export trade. Australian Commonwealth Government, retrieved from http://www.daff.gov.au/animal-plant-health/welfare/export-trade
- Fleming, P., Corbett, L., Harden, R. and Thomson, P. (2001). Managing the impacts of dingoes and other wild dogs. Book, published by Australian Bureau of Rural Statistics.
- Burns, G. L., & Howard, P. (2003). When wildlife tourism goes wrong: a case study of stakeholder and management issues regarding Dingoes on Fraser Island, Australia. Tourism Management, 24(6), 699-712.
- Department of Fisheries (2013). New measures to combat WA shark risk. Western Australian Government. Retrieved from http://www.fish.wa.gov.au/About-Us/Media-releases/Pages/New-measures-to-combat-WA-shark-risks.aspx
- Essendelft, W. V. (2005). Dingo fence (picture). Retrieved from http://www.dingofence.com/realdf.php